Platelets are megakaryocyte-derived enucleated blood cells that are primarily responsible for hemostasis (staunching bleeding) in the body. Platelets render hemostasis by marginating across bulk blood flow towards the vascular injury site, adhering to the site via molecular interactions with specific proteins (e.g. vWF and collagen), undergoing activation as an effect of adhesion-induced signaling, secreting platelet agonists (e.g. ADP and thrombin) to further locally activate other platelets and promoting aggregation of these activated platelets via inter-platelet bridging mediated by ligand interactions with platelet surface integrins and selectins. The aggregation of active platelets at the vascular injury site (primary hemostasis) also presents negatively charged phospholipids on the active platelet surface, on which the coagulation factors can co-localize and trigger the coagulation cascade to ultimately form fibrin clot from fibrinogen (secondary hemostasis). 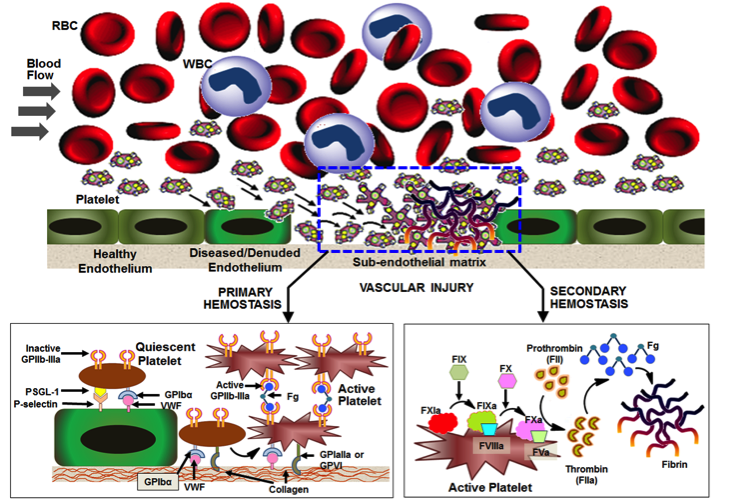 See Figure 1 (Platelet’s natural mechanisms of margination, adhesion and aggregation to render Primary Hemostasis and facilitate Secondary Hemostasis) for a mechanistic description of these events. Heavy blood loss due to trauma or during surgery, as well as, congenital or drug-induced effects on bone marrow and blood components can affect platelet number and functions, which in turn can affect a person’s ability to undergo hemostasis when needed. In such bleeding risk scenarios, current clinical strategies are to carry out natural platelet transfusions. However, natural platelet based products suffer from low donor availability, high risk of pathologic contamination and biologic side-effects, and a very low shelf life (< 7 days). Therefore there is a significant clinical interest in ‘synthetic platelet’ systems that render platelet-mimetic hemostasis, while allowing advantages of large scale production,low contamination risks and reduced side-effects. To this end, our lab is designing ‘synthetic platelets’ inspired by the natural mechanistic cues of platelet’s hemostatic action. Specifically our lab is investigating a unique design where platelet’s margination-influencing physical parameters (e.g. size, shape, flexibility etc.) and hemostasis-influencing biochemical interactions (e.g. vWF-binding, collagen-binding, integrin-mediated aggregation etc.) are integrated on biomaterial-based particle platforms made from lipids, natural polymers and synthetic polymers. Our design is the first to investigate such modular integrative approach in platelet mimicry. The research is currently funded by NIH (http://engineering.case.edu/NIH-platelet) and has collaborations with The Cleveland Clinic Foundation and University of California, Santa Barbara. You can read more about this in the Publications and the News.
See Figure 1 (Platelet’s natural mechanisms of margination, adhesion and aggregation to render Primary Hemostasis and facilitate Secondary Hemostasis) for a mechanistic description of these events. Heavy blood loss due to trauma or during surgery, as well as, congenital or drug-induced effects on bone marrow and blood components can affect platelet number and functions, which in turn can affect a person’s ability to undergo hemostasis when needed. In such bleeding risk scenarios, current clinical strategies are to carry out natural platelet transfusions. However, natural platelet based products suffer from low donor availability, high risk of pathologic contamination and biologic side-effects, and a very low shelf life (< 7 days). Therefore there is a significant clinical interest in ‘synthetic platelet’ systems that render platelet-mimetic hemostasis, while allowing advantages of large scale production,low contamination risks and reduced side-effects. To this end, our lab is designing ‘synthetic platelets’ inspired by the natural mechanistic cues of platelet’s hemostatic action. Specifically our lab is investigating a unique design where platelet’s margination-influencing physical parameters (e.g. size, shape, flexibility etc.) and hemostasis-influencing biochemical interactions (e.g. vWF-binding, collagen-binding, integrin-mediated aggregation etc.) are integrated on biomaterial-based particle platforms made from lipids, natural polymers and synthetic polymers. Our design is the first to investigate such modular integrative approach in platelet mimicry. The research is currently funded by NIH (http://engineering.case.edu/NIH-platelet) and has collaborations with The Cleveland Clinic Foundation and University of California, Santa Barbara. You can read more about this in the Publications and the News.
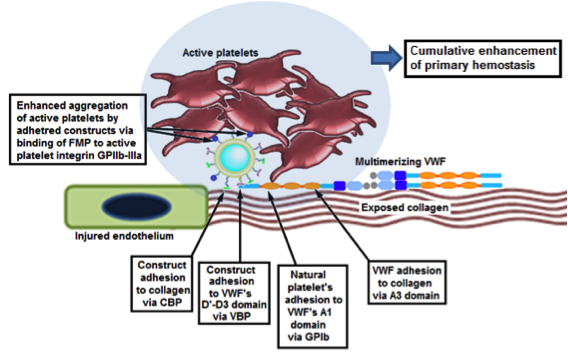 Design of liposomal synthetic platelets that adhere to vWF and collagen and promote injury site-selective platelet aggregation Design of liposomal synthetic platelets that adhere to vWF and collagen and promote injury site-selective platelet aggregation |
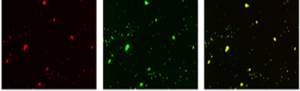 Particle Adhesion Platelet Aggregation Colocalization Particle Adhesion Platelet Aggregation Colocalization |
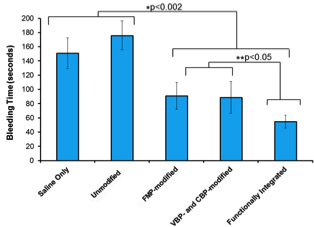 Integrative design of ‘Synthetic Platelets’ reduce bleeding in animal model by ~70% Integrative design of ‘Synthetic Platelets’ reduce bleeding in animal model by ~70% |