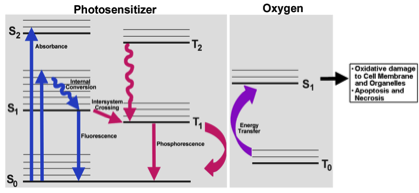
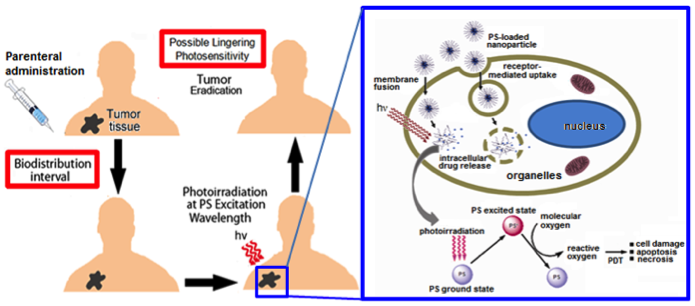
Photodynamic Therapy (PDT) is a promising cancer treatment strategy that can potentially reduce the risks of systemic toxicity, collateral healthy tissue damage and surgical trauma and tissue debilitation, especially when repeated treatment becomes necessary because of the aggressive nature of the cancer. PDT involves three components: a photosensitizer (PS) drug, a specific wavelength of drug-activating light and oxygen. Light activation of a photosensitizer results in energy transfer cascades that ultimately yield cytotoxic reactive oxygen species, which can then render apoptotic and necrotic cell death. Since the first modern demonstration of harnessing this mechanism to kill tumor cells by Dougherty et al. in 1978, PDT has undergone extensive investigations in cancer treatment. A major advantage of PDT over conventional chemotherapy is that the PS itself is minimally toxic in the absence of light and hence PS accumulation in non-specific tissues confers minimal systemic toxicity. Furthermore, compared to radiotherapy, the activating light is non-ionizing and hence its effect on tissues without the PS drug is not harmful. As a result, PDT has the potential to be repeated safely if needed without the risk of harming neighboring healthy tissue. This makes PDT very attractive for tumors where loco-regionally recurrent treatment may be needed. By virtue of these characteristics, PDT can offer the possibility of dual-selectivity in cancer therapy by developing technologies that ensure PS accumulation selectively in the tumor and light irradiation selectively at the tumor site. The clinical interest in PDT is exhibited by health agency approvals with the PS formulations Photofrin®, Levulan® and Visudyne® in the US, various other formulations (Foscan®, Photosense®, Metvix®) in other countries, and ongoing clinical trials. To this end, our research is focused on cell-targeted nanoformulations of silicon-based photodynamic agents that can be preferentially accumulated within the tumor stroma via the enhnaced permeation and retention (EPR) mechanisms and further internalized within the tumor cells via active receptor-mediated cytoplasmic uptake mechanisms. Currently we are carrying out PDT Nanomedicine research in Head-and-Neck, Cervical, Breast and Skin Cancer areas. We also have collaborative research in the area of hand-held optical imaging instruments that can detect the tumor-uptake of PDT nanomedicine and allow targeted delivery of photoactivating lasers to selectively kill the tumor cells (i.e. image-guided PDT). Another collaborative research area is the utilization of nanomedicine platforms that allow simultaneous delivery of oxygen and PS molecules selectively to the tumor.
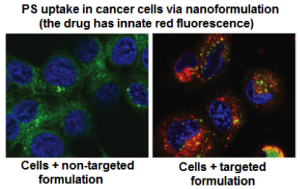 Uptake of PS molecule Silicon Phthalocyanine 4 (Pc 4) in head-&-neck cancer cells (SCC-15) when delivered via non-targeted versus cell-targeted nanomedicine platform. Receptor-mediated internalization of the targeted formulation leads to significant cellular uptake
Uptake of PS molecule Silicon Phthalocyanine 4 (Pc 4) in head-&-neck cancer cells (SCC-15) when delivered via non-targeted versus cell-targeted nanomedicine platform. Receptor-mediated internalization of the targeted formulation leads to significant cellular uptake