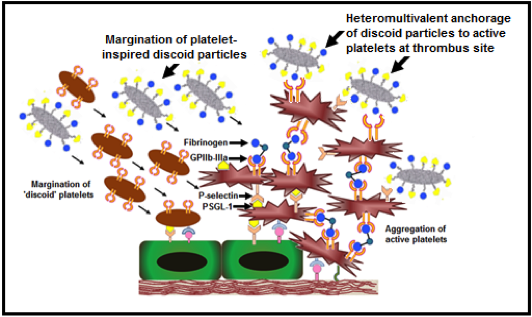
Dysregulated hemostatic mechanisms often lead to hyperaggregation of activated platelets and overaction of coagulation events, ultimately leading to occlusive clot formation. Such occlusive clotting is a primary characteristic in many vascular disease conditions, leading to ischemia, unstable angina, myocardial infarction, stroke etc. Such conditions lead to significant tissue morbidity and mortality, and hence dissolving or removing the clot rapidly to re-establish blood flow to critical tissues and organs remains a clinical mainstay in treating these conditions. The mechanical strategies for clot removal include thrombectomy or angioplasty (with or without stenting) which are invasive and can cause secondary injuries and thrombotic events, leading to re-occlusion. The surgical strategies to re-establish blood flow mainly involve bypass grafting, that often has to address challenges of graft patency and occlusion. In this framework, stand-alone or adjunctive pharmacotherapeutic strategies are often used that involve systemic administration of clot-busting drugs (e.g. tissue plasminogen activator or tPA), anti-coagulants (e.g. heparin and its derivatives) and anti-proliferatives (e.g. sirolimus). Systemic delivery of these agents often lead to low circulation half-life, plasma-induced deactivation or rapid clearance of these drugs, as well as, indiscriminate drug action and non-specific sites leading to harmful side effects like hemorrhage. Consequently, there is a significant clinical interest in technologies that can ensure delivery of these agents specifically at the vascular disease or thrombo-occlusion site. To this end, we are utilizing blood platelets as a design paradigm to develop drug delivery vehicles that can actively anchor onto vascular disease sites or sites of thrombo-occlusion, and deliver drugs and imaging agents to these sites for targeted action. The rationale behind the ‘platelet paradigm’ is the fact that active platelets are spatio-temporally present at various stages of vascular disease progression and thrombo-occlusion, and have the innate ability to marginate to these disease or clot sites, bind actively via specific molecular interactions and secrete a variety of cytoplasmic granule contents to modulate clotting events. Figure 1 shows a simple schematic of platelet involvement in occlusive thrombus formation and the representative molecular interactions that inspire the design of drug delivery vehicles by utilizing the platelet-relevant interactions.
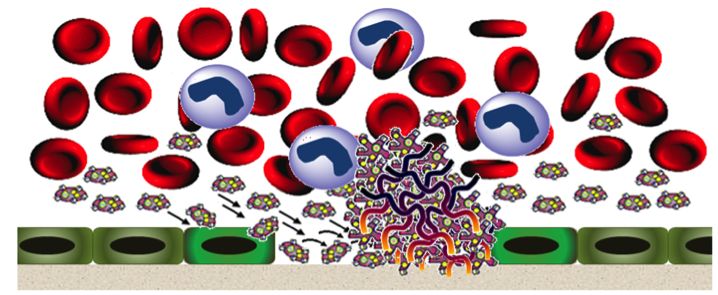 Figure 1. Dysregulated hemostasis leading to platelet-rich thrombus formation Figure 1. Dysregulated hemostasis leading to platelet-rich thrombus formation |
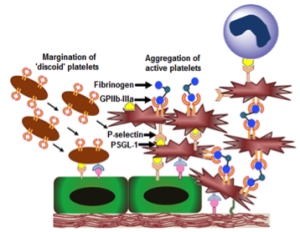 Molecular interactions of platelets in thrombus formation Molecular interactions of platelets in thrombus formation |