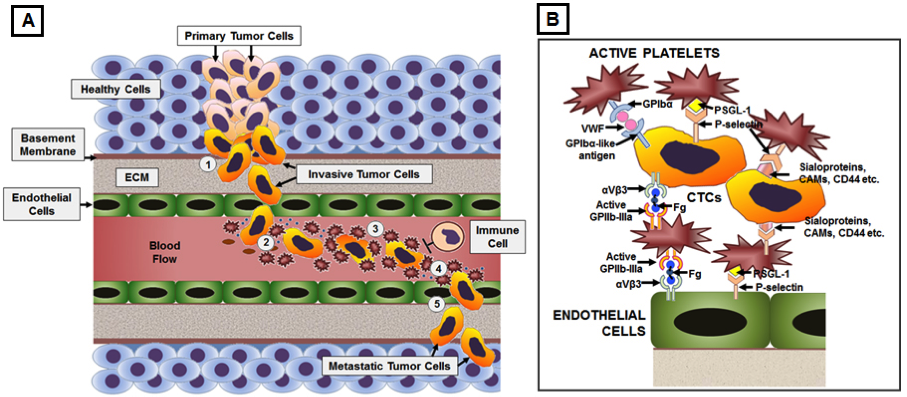
Disseminated metastatic cells that intravasate into circulation (called circulating tumor cells or CTCs) are expected to have poor survival rates due to lack of matrix adhesion, increased fluid mechanical stresses of blood circulation and action of immune cells. Yet, some of these cells are found to effectively avoid anoikis, undergo epithelial-to-mesenchymal transformation en route to intravasation, avoid immune surveillance in circulation and undergo arrest or adhesion at distal site vasculature to form metastatic colonies. Several research studies have demonstrated that binding interactions with active platelets and action of platelet-derived secretome may play a significant mechanistic role in facilitating these processes. Active platelets can promote epithelial-to-mesenchymal transition in cancer cells, for example, by secretion of
TGFβ and activation of TGFβ/Smad and NF-kB pathways. Active platelets can secrete pro-angiogenic cytokines (e.g., VEGF-A, PDGF etc) and proteases (e.g., MMP-2, MMP-9 etc) to influence tumor vascularization, matrix and basement membrane degradation, cancer cell migration and intravasation. Following intravasation, tumor cells can induce further platelet activation (e.g., via tissue factor pathway) and aggregation around themselves to form a “platelet cloak” to stay protected from immune surveillance in circulation. Subsequently, active platelets can facilitate the arrest of circulating tumor cells under dynamic blood flow conditions onto the vascular wall at secondary sites, and mediate endothelial retraction, matrix degradation, and extravasation of CTCs. Active platelet secretome and platelet-mediated recruitment of leukocytes can regulate inflammation, matrix remodeling, and angiogenesis to promote and maintain the metastatic microenvironment. Based upon such cues, the overall goal of this research is to elucidate the mechanistic role of platelets in mediating pathways of tumor metastasis and then utilize these interactive mechanisms on synthetic platforms for targeted drug delivery, as well as, cell-capture/detection technologies for diagnosis and treatment of cancer metastasis. The platelet-inspired approach has the potential to lead to highly novel routes for targeted therapy of metastasis where current targeting approaches become limited due to receptor heterogeneity and microenvironment variations. The cell capture technologies will be directed towards development of high-throughput systems for sampling patient blood as a diagnostic assay for metastatic risks.
Figure 1. Mechanistic involvement of platelets with CTCs in facilitating hematologic pathways of metastasis
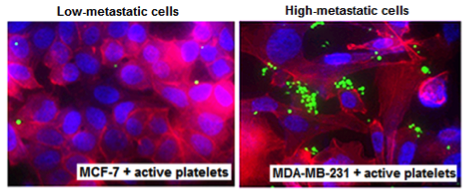 Active platelets (green) show enhanced interactions with highly metastatic cancer cells compared to low-metastatic ones. The cancer cells are stained with DAPI (blue nucleus) and phalloidin (red cytoplasm) Active platelets (green) show enhanced interactions with highly metastatic cancer cells compared to low-metastatic ones. The cancer cells are stained with DAPI (blue nucleus) and phalloidin (red cytoplasm) |
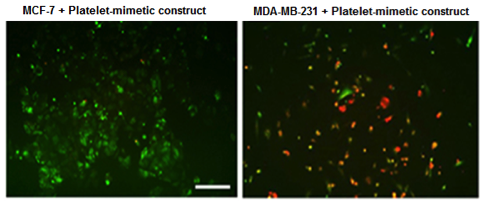 Nanoconstructs having platelet-mimetic interaction capabilities show enhanced binding to pro-metastatic cells compared to low metastatic cells. The constructs are red fluorescent while the cells are green fluorescent Nanoconstructs having platelet-mimetic interaction capabilities show enhanced binding to pro-metastatic cells compared to low metastatic cells. The constructs are red fluorescent while the cells are green fluorescent |
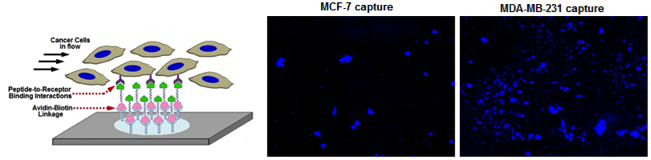 Microfluidic surfaces presenting orthogonal brushes of ligands that promote platelet-inspired binding interactions with metastatic cells enable enhanced capture of highly metastatic cells compared to low metastatic cells Microfluidic surfaces presenting orthogonal brushes of ligands that promote platelet-inspired binding interactions with metastatic cells enable enhanced capture of highly metastatic cells compared to low metastatic cells |
|