Interaction of biomedical devices and implants with the living tissue is largely mediated by surface chemistry and surface morphology. For example, for blood-contacting devices (vascular grafts, wound healing systems, dialysis membranes etc.), protein adhesion, denaturation and retention on the device surface dictates the long term favorable or unfavorable cellular interactions with the device. For bio-inspired devices or tissue engineered constructs, often specific cell-material interactions are required for intended property and functions. To this end, our laboratory develops and studies variety of physical and chemical surface modification technologies for preventing or promoting specific protein-material and cell-material interactions. These strategies involve glycocalyx-inspired saccharide-based interfacial coatings (collaboration with Prof. Roger Marchant), as well as, polyethylene glycol-based and polyelectrolyte-based hydrophilic surface modification of implants and extra-corporeal devices, for reduced thrombogenicity, increased cell-specificity, and localized delivery of bioactive agents. The research also spans creation of coated microfluidic surfaces for protein-specific and cell-specific biointeractions, with a vision for diagnostic technologies.
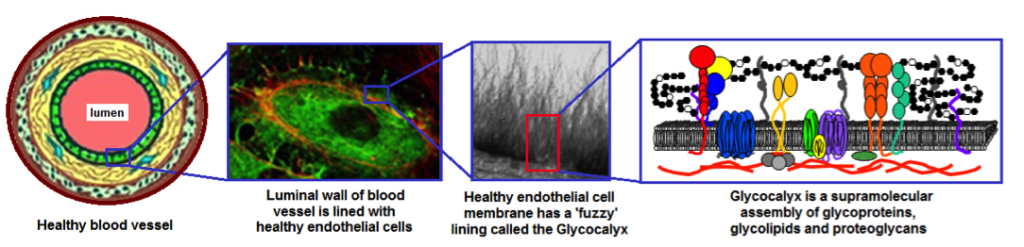
Figure 1. Hierarchical representation of Glycocalyx lining of healthy endothelium on the luminal wall of blood vessels. The Glycocalyx facilitates specific biointeractions for cellular communications and prevents non-specific protein adsorption and cellular adhesion by virtue of maintaining a hydrophilic steric barrier close to the endothelial surface. Surface modification of biomaterials inspired by this Glycocalyx function, for example, by physisorption or chemical conjugation of hydrophilic polymers (e.g. carbohydrates, PEG, polyelectrolytes etc.) can lead to reduced thrombogenicity and increased blood compatibility. The surface-modification strategies can also incorporate biochemical motifs for facilitating specific cell-cell and cell-material interactions (e.g. endothelialization).
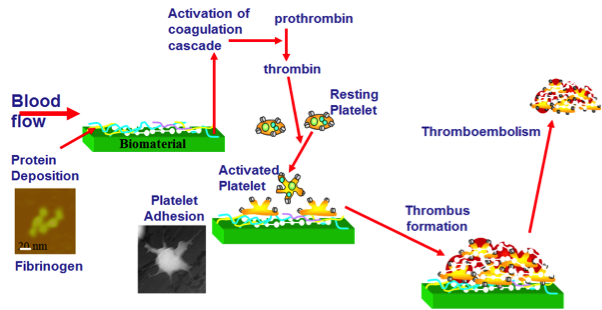 Figure 2. A schematic representation of the mechanism of protein adsorption and subsequent adhesion/activation of platelets on biomaterial surfaces leading to clot formation, device failure and further thromboembolism risks Figure 2. A schematic representation of the mechanism of protein adsorption and subsequent adhesion/activation of platelets on biomaterial surfaces leading to clot formation, device failure and further thromboembolism risks |
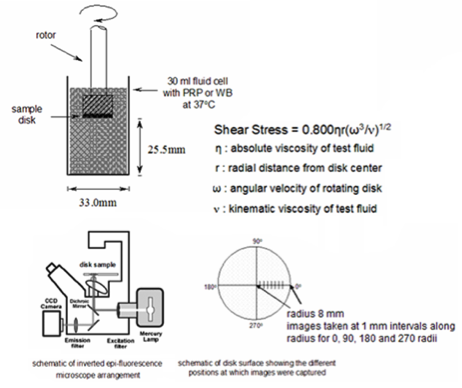 Figure 3. A Rotating Disc (RD) apparatus for in vitro testing of biomaterial samples (in disc form) in whole blood, to determine protein adsorption and platelet adhesion/activation under physiologically and pathologically relevant shear rates Figure 3. A Rotating Disc (RD) apparatus for in vitro testing of biomaterial samples (in disc form) in whole blood, to determine protein adsorption and platelet adhesion/activation under physiologically and pathologically relevant shear rates |
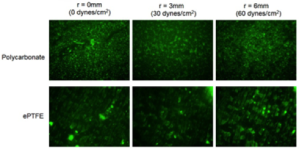 Representative images of adhered activated platelets (green fluorescence) on some biomedical polymers tested using the RD apparatus for various shear values Representative images of adhered activated platelets (green fluorescence) on some biomedical polymers tested using the RD apparatus for various shear values |
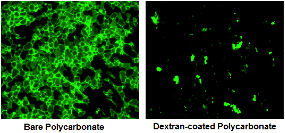 Reduction in platelet adhesion on biomaterial surface following coating with glycocalyx-inspired carbohydrate polymers Reduction in platelet adhesion on biomaterial surface following coating with glycocalyx-inspired carbohydrate polymers |
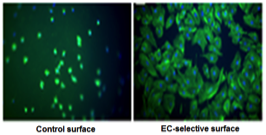 Stable endothelialization of biomaterial surfaces using coatings that prevent platelet adhesion but allow EC adhesion Stable endothelialization of biomaterial surfaces using coatings that prevent platelet adhesion but allow EC adhesion |